
LentiSure™: Plug & Play Robust and Scalable, Lentivirus Manufacturing Platform
Lentivirus-based process platform
With an increasing number of cell therapies entering pre-clinical and clinical trials, the demand for lentiviral vectors is higher than ever, and with this the need for safe and efficient lentivirus manufacturing.
LentiSure™, a large-scale lentivirus manufacturing platform designed by Yposkesi, an SK pharmteco company, provides the premium quality grade lentiviral vectors you need to assure your clinical development and commercial cell and gene therapy pipeline. LentiSure’s robust, scalable, and plug & play-optimized processes enable you to accelerate the time to market of your cell and gene therapies and facilitate better patient outcomes. You obtain a safe and effective product through LentiSure’s high productivity and established analytical methods. LentiSure is the choice for high titers, transduction efficiency, reliability, and accessing scalable production processes with optimized purification techniques.
Learn more about the advantages of LentiSure, a lentivirus manufacturing platform designed for both adherent and suspension processes and developed with single-use equipment.
LentiSure gives you access to a time-efficient and cost-effective streamlined environment, enabling you to commercialize your cell and gene therapies within your target timelines. It is designed for both adherent and suspension processes.
Plug and play
LentiSure is a fully integrated platform. This means that LentiSure is fully integrated into the manufacturing structure. Everything is organized with optimized processes; all Yposkesi needs to get started is your transgene and to run some standard feasibility studies. LentiSure is proven and optimized by our experts to deliver high-quality lentiviruses for your cell and gene therapies. On top of that, all the analytics are integrated within the platform.
Productivity and Scalability
LentiSure is optimized to deliver high-quality lentiviral vectors in a productive and scalable manner. Produced with Yposkesi’s LentiSure platform, lentivirus yields at harvest are high: up to 5.9×107 IG/mL, and no loss of titer is observed between a 250 mL batch or a 200 L batch, meaning that high titers will be maintained while scaling up your production. This has been confirmed using several scales and different transgenes.
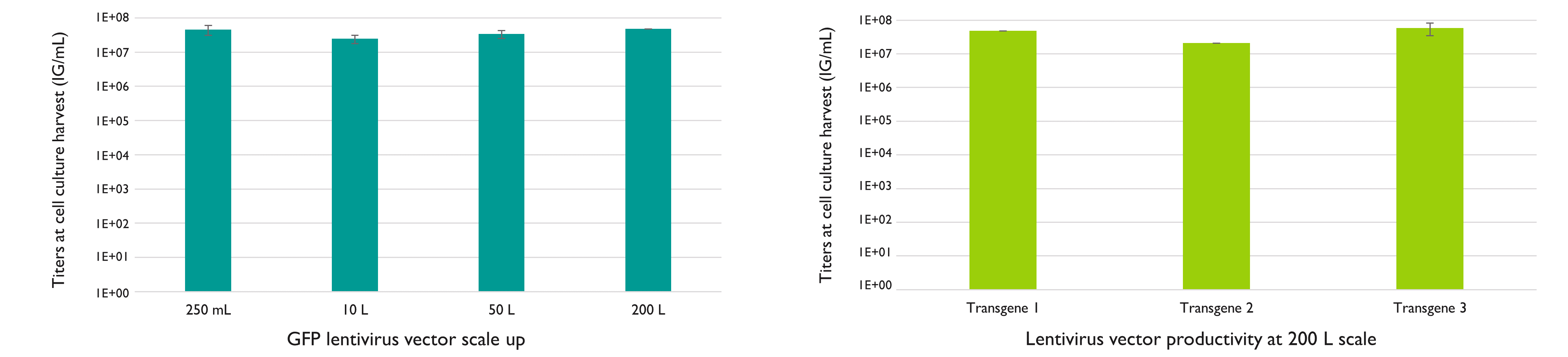
Time-savings
Designed to be time and cost-effective, reducing man-hours and increasing output, LentiSure can guarantee lot readiness to batch results in six months for current Good Manufacturing Practices (cGMP) batches and even less for non-cGMP.
Analytics
Because lentivirus manufacturing cannot be separated from analytics, LentiSure is equipped with proven and optimized analytical methods. Recognized by the leading regulatory authorities, 95% of our analytical tests are performed in-house and a fully qualified external network is well-established for specialized testing. Click here to learn more about our analytical methods.
Expertise
The experts behind LentiSure make the platform effective. Yposkesi has more than 30 years of experience in lentivirus manufacturing. Our talented workforce of highly-qualified experts has already delivered dozens of successful lentivirus batches. LentiSure is optimized and automated, to ensure the following:
- Decreased number of staff interventions
- Reduced costs
- Increased efficiency
Robustness
With a high success rate of >95%, LentiSure has proven capacity to produce high-quality grade cGMP lentiviruses.
Safety
As an SK pharmteco company, Yposkesi adheres to the motto for ‘Safety First, Quality Always’ where the patient is at the heart of our manufacturing development and safety is our priority. LentiSure is in full compliance with Yposkesi’s robust and proven Contamination Control Strategy (CCS).
CMC and Regulatory Support
From the beginning of your project, a dedicated Chemistry, Manufacturing, and Control (CMC) Project Manager will be assigned to your project and will become your key point of contact. Ensuring you are kept up to date with every step of the project’s progress, you will get real-time updates from Yposkesi’s dedicated team of experts. They are responsible for ensuring your project is on track for timelines, quality requirements, and costs.
The project manager will:
- Organize a kick-off meeting: to review your project content (work package definition, technical content, pre-requisites, expected outcomes, deliverables, milestones, etc.), timelines, presentations from the dedicated Yposkesi teams and if necessary, a risk assessment plan;
- Organize regular team meetings: frequency is adapted depending on your project phase and needs. During each project team meeting, driven by the project manager, a project update is performed with a review of each work package for current and next steps including technical content, timelines, and quality.
Frequently Asked Questions (FAQs)
The manufacturing of lentivirus involves several steps:
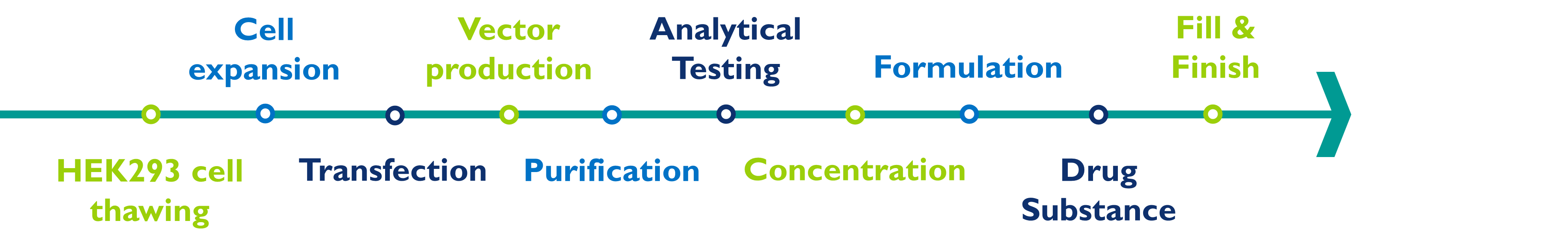
HEK293T cell thawing and expansion: To thaw and then expand the number of cells in the culture, this step can be done with an adherent or a suspension bioreactor.
Vector production: The lentiviral vector is transfected into the cells, which allows the production of large quantities of virus particles. This is usually done in multi-layer cell culture flasks or suspension bioreactors using chemical transfection reagents.
Virus purification: The virus particles are then purified from the cell culture harvest using techniques such as filtration, chromatography, and tangential flow filtration.
Analytical testing: The purified virus is then tested to ensure that it is pure and free of contaminants. This can be done using techniques such as biochemical assays, molecular biology tests, protein assays, and potency assays.
Virus concentration: The purified virus is then concentrated to the desired concentration using techniques such as ultra- or tangential flow filtration.
Formulation: The concentrated virus is then formulated with excipients.
Drug substance: The final formulated bulk drug substance can be frozen and stored for later drug product manufacturing or can be onward processed to the drug product manufacturing step.
Fill and finish: The formulated concentrated virus is sterile filtered and filled into appropriate containers for storage and use. It is important to follow cGMP when producing lentivirus to ensure that the final product is safe and effective for use in humans.
Yes, LentiSure was developed using single-use equipment. Working with single-use equipment allows our experts to work in a closed environment, reducing the risk of cross-contamination, the need for equipment cleaning and steaming processes, and providing consistent batch-to-batch results. On top of that, it enables our Scientists to provide a fast change-over time between batches, improving the cost-efficiency of LentiSure.
Yes, to avoid variability and contaminants in cell culture systems, LentiSure is equipped with serum-free media for the suspension platform, and no animal products are used within the platform. Using chemically defined media allows us to manufacture lentivirus with consistency and contamination control.
It is quite standard when a cell and gene therapy developer wants to access LentiSure, that we will start with a standard feasibility study to optimise productivity, followed by the manufacturing of technical batch and clinical batches, and associated analytics.
Within LentiSure, we use 3rd generation lentivirus production system. This generation of vectors allows us to:
- Control the gene expression, because they have regulatory elements such as promoters or enhancers that can be used to control the expression of the transgene
- Increase the safety, because 3rd generation lentiviral vectors include safety features such as self-inactivating (SIN) elements or deletions in viral genes to reduce the risk of insertional mutagenesis or other unwanted effects
- Increase the efficiency, because they include modifications that improve the efficiency of transduction or gene expression in target cells
- Allow versatility, because 3rd generation lentiviral vectors are designed to be flexible and adaptable, allowing them to be used in a wide range of applications and cell types
- Yposkesi has its own cGMP E.coli cell banks for 3rd generation lentivirus plasmids and is able to supply these to our customers.
Yes, a platform for AAV manufacturing, AAVelocity™ is also available at Yposkesi. Learn more about the AAV platform by clicking here.
Yes, because we know that science is constantly evolving, we are always working on innovating and improving our platforms. Learn more about our innovation programs by clicking here.


