Efficient transduction of T cells using a suspension-based lentiviral vector system
A. Parcellier, A. Larbi, E. Neveu, A. Auffret-Cariou, C. Rousseaux and S. Charrier.
Gene transfer technology using lentiviral vectors (LV) is one of the most promising therapeutic approaches for cancer immunotherapy (CAR-T or TCR cells). However, an important manufacturing challenge is the industrialization of lentiviral vector production, especially given the potentially larger quantity/volume requirements for these new applications. Therefore, we have developed and optimized LentiSure™: a new suspension scalable lentiviral vectors manufacturing process based on quadruple plasmid transient transfection of HEK293T cells grown in suspension in bioreactors in serum-free conditions (50L- 200L scale). The cell culture harvest was purified by ion-exchange chromatography and concentrated by tangential-flow filtration. The overall yield of manufactured lentiviral vector was higher than that obtained with an adherent process for a comparable titre. Final vector was characterized for infectious viral titre (IG/mL), particle content (ng p24), and process related impurities (protein and DNA). We then evaluated the efficiency of this new lentiviral vector in parallel to a vector produced by an adherent manufacturing process. Results showed a comparable transduction efficiency of Jurkat cells and activated T cells with lentiviral vectors produced by both adherent and suspension manufacturing processes, with a good correlation between multiplicity of infection (MOI), vector copy number and protein expression. We also demonstrated similar behaviour of T cells transduced with a lentiviral vector produced either by an adherent or the new suspension processes. In conclusion, lentiviral vectors manufactured by this new scalable suspension manufacturing process results in a larger quantity (up to 200L) of infectious and physical particles with a similar transduction efficiency in T cells, to a lentiviral vector produced by conventional methods.
Suspension rLV Process
The third generation of SIN lentiviral vectors, used for clinical trials are produced by transient transfection with four plasmids: one plasmid encoding the Gene of interest (GOI) and flanked by deleted long terminal repeat (LTR) sequences and helper plasmids encoding the Vesicular Stomatitis Virus G (VSV-G) envelope, and the gene gag-pol and rev genes. The manufacturing process is based on quadruple plasmids transfection of suspension-adapted HEK293T cells grown in bioreactors in serum-free conditions, purified by ion-exchange chromatography and concentrated by tangential-flow filtration steps, enabling concentration of the vector about 250 to 500-fold volume.
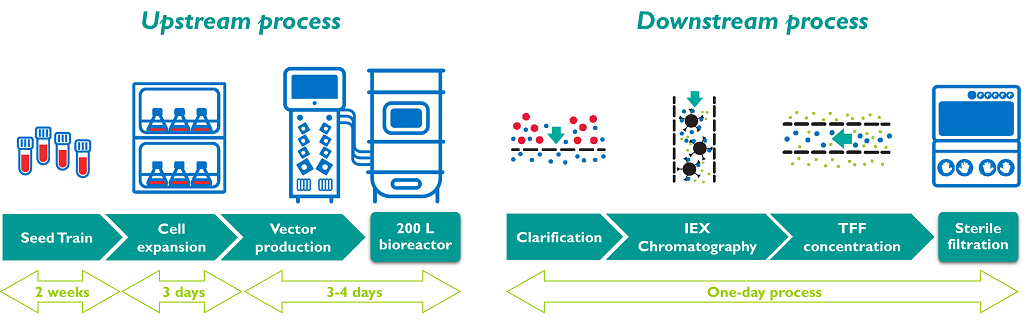 Lentisure™: Yposkesi’s lentiviral manufacturing platform unit operations
Lentisure™: Yposkesi’s lentiviral manufacturing platform unit operations
Lentiviral vector: product comparison between suspension and adherent systems
Lentiviral vectors used in gene therapy are conventionally manufactured by calcium phosphate transfection of adherent HEK293T cells and require fetal bovine serum in the culture media. Because the presence of the animal-derived component in the culture constitute a safety risk that limits the GMP compliance of the process, a scalable lentiviral vector has been developed in Yposkesi to be compatible with industrial pharmaceutical applications in bioreactors with a serum-free media from 3L until 200L.
Infectious and physical titration
At the Drug Substance (DS) step, infectious titers of lentiviral vectors produced from adherent and suspension process were determined by quantitative PCR targeting genomic DNA to quantify pro-viral sequences in the LV Psi region normalized to the human albumin gene, and then expressed as infectious genomes (IG) per mL. The physical titer was expressed as gag p24 antigen quantity (ng p24) per mL and was determined by ELISA.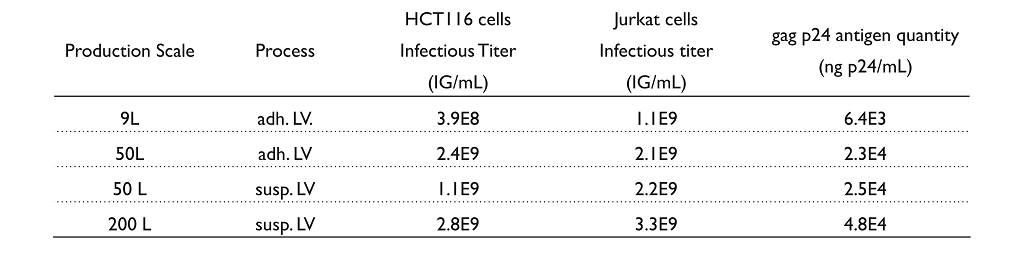
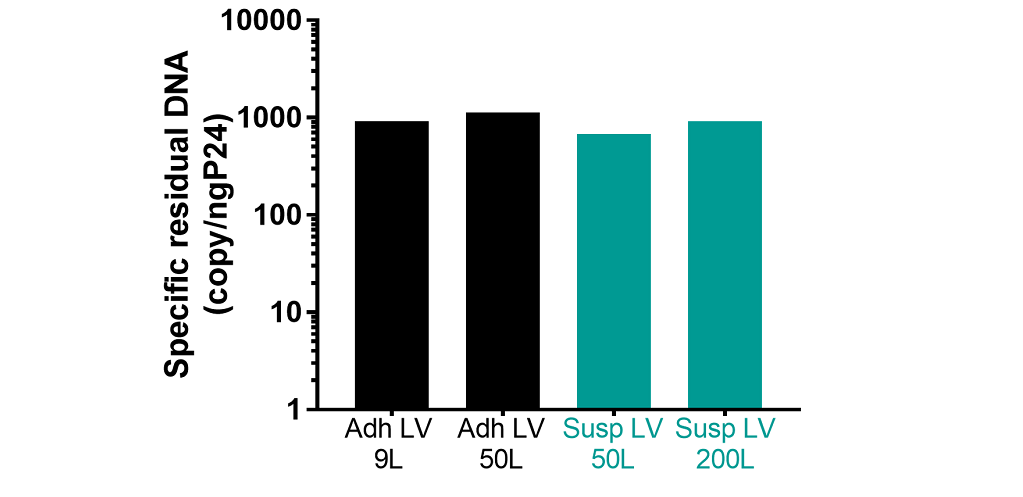
Residual plasmid impuritiesDNA plasmid impurities were quantified on DS product by qPCR specific neomycin phosphotransferase gene.
|
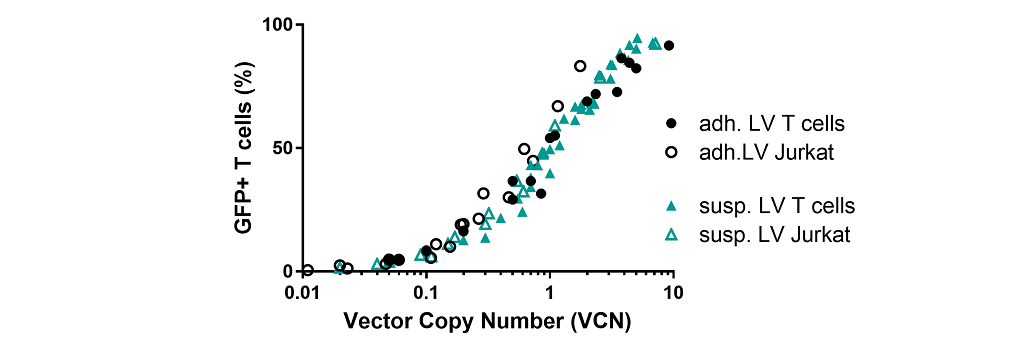
Transduction efficiency on T cell linesSeveral lentiviral concentrations were used for the evaluation of LV vectors transduction of Jurkat T cells and activated CD4+ and CD8+ T cells.
|
- Characteristics of lentiviral vectors produced with a suspension system are consistent with those produced with an adherent system.
- Correlation between pro-viral integration (VCN) and transgene expression is similar for both vectors.
Behaviour of transduced T cells
Because the functional profiles of T cells are dependent on memory/effector molecules, stimulatory/proliferative cytokines or chemokines, we have also evaluated the potential impact of lentiviral vectors produced by both systems on the T cells responses after transduction and expansion. T cells were activated by MACS GMP T Cell TransactTM reagent (Day 0) and then transduced (Day 1) by lentiviral vectors concentration at 2E6 and 5E6 IG/mL (corresponding to MOI 2 and 5). T cell subsets and cytokine secretion levels were determined by flow cytometry.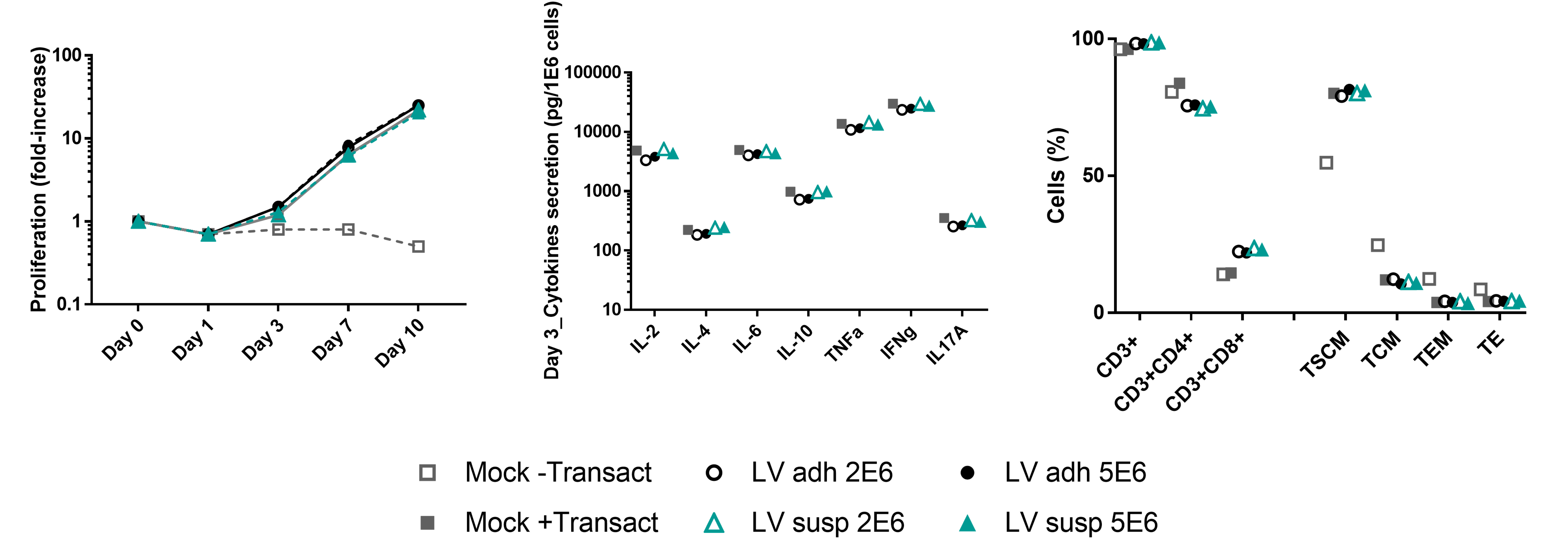
- T cells showed similar proliferation and cytokine secretion profiles on Day 3, and T cell subsets at Day 10, regardless of the production system used to generate the lentiviral vectors.
TSCM: Stem Cell-like Memory T cells (CD3+CD45RA+CD62L+CD95+); TCM: Central Memory T cells (CD3+CD45RA-CD62L+CD95+); TEM: Effector Memory T cells (CD3+CD45RA-CD62L-CD95+); TE: Effector T cells (CD3+CD45RA+CD62L-CD95+).
T cell transduction procedure using Lentiviral vector produced by suspension system
According to the infectious titer on Jurkat T cells, we have evaluated the transduction efficiency (VCN and GFP+ T cells) in order to define the lentiviral concentration necessary to reach higher than 50% of transduced cells in large scale by using CliniMACS prodigy and the TCT process (10 days).
Parameters of transduction of T cells
Several dilutions of lentiviral vectors produced by the suspension production system were used to transduce CD4+ and CD8+ T cells, 24h post-stimulation by TransactTM reagent.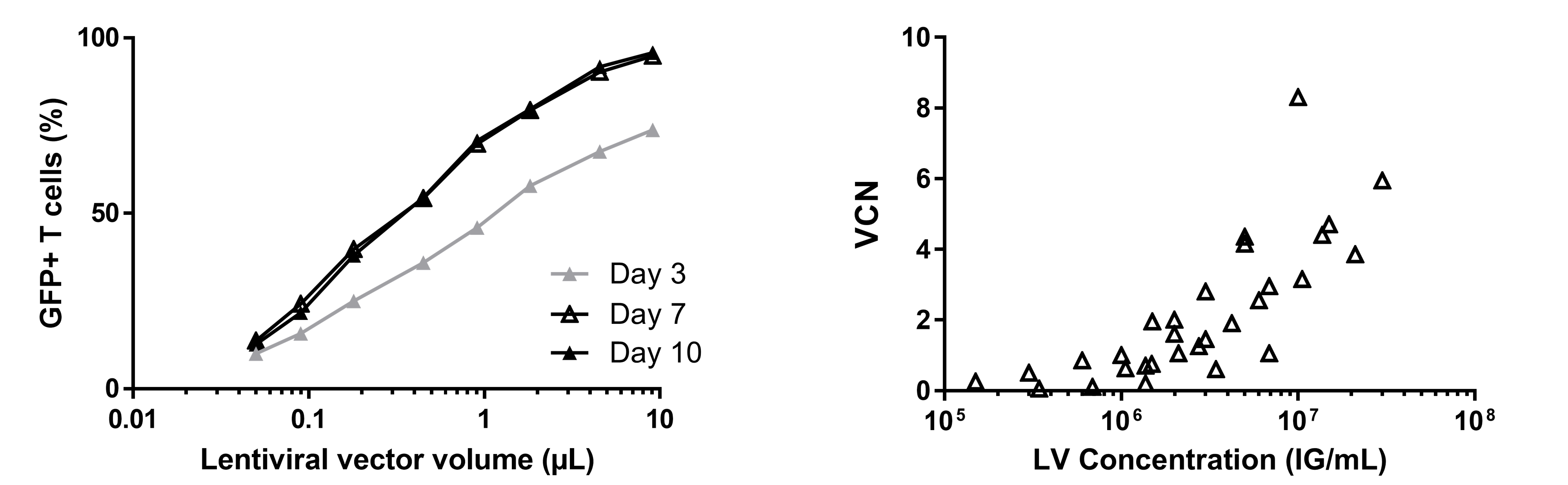
- A stabilization of transgene expression was observed from seven days post-transduction of T cells.
- The vector concentration range used was between 1E6 and 5E6 IG/mL (MOI 1 to 5) to reach less than 2-3 copies of integrated vectors in T cells.
Automated T cell Transduction (TCT) process
The manufacturing process of gene-modified T cells requires the development of closed systems in order to enable automation and standardization. Large scale TCT procedures developed on the CliniMacs Prodigy were performed with T cells from two different healthy donors using a suspension lentiviral vector concentration of 3E6 IG/mL.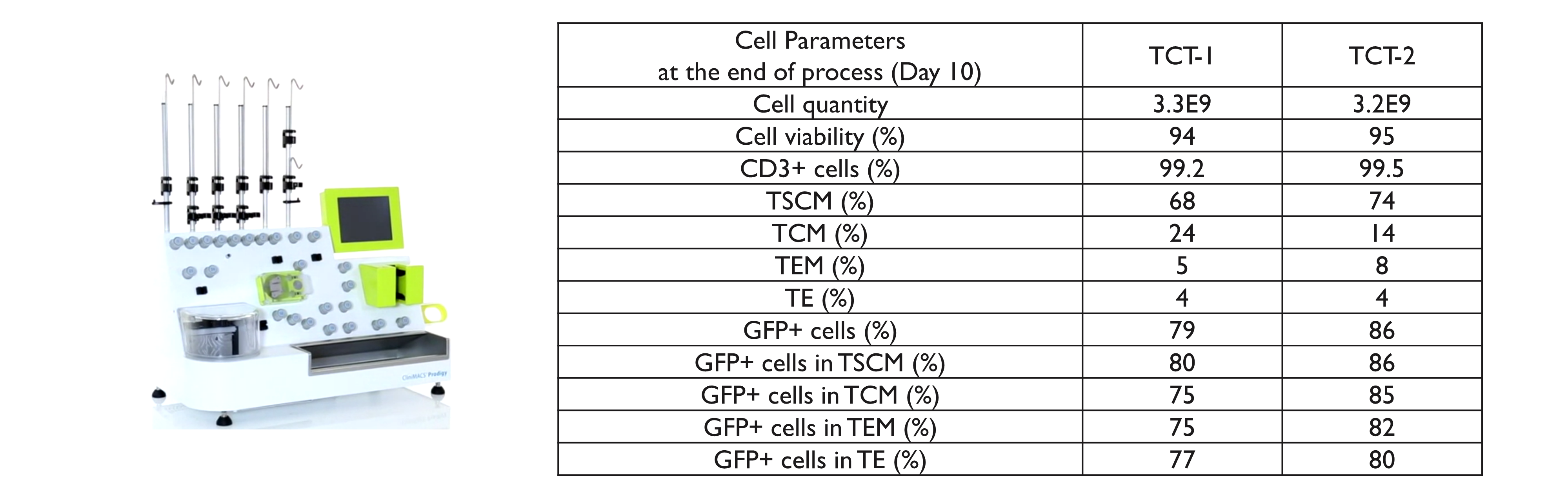
- These results confirm the feasibility to use the lentiviral vectors produced by suspension systems at large scale to manufacture gene-modified T cells.
Conclusion
In this work, it was important to evaluate the quality of these new lentivectors on the transduction efficiency on target cells and mainly, in the context of the genetically modified T cells manufacturing. We have shown the feasibility to use lentiviral vectors produced by our new scalable suspension system, LentiSure™, with comparable transduction efficiencies and T cell functionality than lentivectors produced by adherent system.
These results are encouraging for the development of new processes for lentiviral production, based on serum-free conditions and bioreactors (50L-200L) and necessary for gene modified cell therapy manufacturing, as regards scalability and regulatory aspects (e.g., animal-free reagents).
This work was supported by CIR (Crédit d’impôt Recherche. FRANCE). The authors would also like to acknowledge Julien Buisset and Emilie Gobbo for their technical support along with the QC and Production teams at Yposkesi and l’Etablissement Français du Sang (EFS Evry, France) for providing the blood samples used for the tests.